A petition to stop FDA misleading doctors about covid-19 vaccines
Experts warn that the FDA is misleading doctors about the safety and efficacy of covid-19 vaccines, denying people the right to informed consent.
American clinicians typically call the folded-up piece of paper inside drug packaging the ‘package insert.’ Others call it the product label.
By law, the notoriously long, fine print prescribing information should contain accurate information about the safety and efficacy of the product.
But the existing product labels for the Pfizer and Moderna mRNA covid vaccines are out of date and omit important information, according to a group of nine experts belonging to the Coalition Advocating for Adequately Labeled Medicines (CAALM).
CAALM recently submitted a formal petition to the FDA asking for the labels on Pfizer and Moderna mRNA vaccines to be updated.
Linda Wastila, professor at the University of Maryland School of Pharmacy and CAALM member says, “If prescribers do not have the most current labelling information, how can they in good faith recommend to a patient that they should get the injection?”
“One of the big problems of the vaccine roll-out has been the lack of informed consent. Millions of people got the injection and have no clue about many aspects of the vaccine,” says Wastila.
If doctors are curious about a specific adverse event, they might look up the FDA or CDC website and conclude there's nothing to be concerned about.
But Wastila says, “By law, the product label must be accurate so having current information on the label is crucial. Part of the reason why people talk about myocarditis is because it’s on the label - it gives it legitimacy."
The 23-page document is compelling.
It meticulously outlines the rationale for each of ten requested efficacy and safety updates, and asks that the FDA communicates these changes via “Dear Health Care Provider” letters.
Wastila says, “In the document, we use the government’s and the manufacturer’s own words and data to show how misleading the public messages have been. It’s eye-opening.”
Other signatories on the petition include Peter Doshi, associate professor at the University of Maryland School of Pharmacy, Caleb Alexander, professor at Johns Hopkins Bloomberg School of Public Health, Vinay Prasad, haematologist-oncologist at the University of California San Francisco, Kim Witczak, leading international drug safety advocate, Marty Makary, public policy researcher at Johns Hopkins University, and Tianjing Li, associate professor at the University of Colorado Anschutz Medical Campus.
Updates on efficacy
The experts state that there is a widespread (but inaccurate) belief that vaccine efficacy against infection and transmission has been established by substantial evidence -- mainly influenced by public statements from President Joe Biden, CDC Director Rochelle Walensky, and former Director of the NIAID, Anthony Fauci.
But the CAALM petitioners explain that FDA evaluation reports and other documents make it clear that evidence is lacking for such claims.
Hence, they’ve requested that the vaccine labels contain “language clarifying that phase III trials were not designed to determine, and failed to provide, substantial evidence of vaccine efficacy against SARS-CoV-2 transmission or death.”
The current Pfizer label also doesn’t mention that the phase III randomised trial – following the Dec 2020 emergency authorisation – showed that efficacy declined following an early peak, and therefore should contain “a clear statement that Pfizer vaccine efficacy wanes after 2 months following dose 2.”
Further, the FDA issued eight emergency use authorisations for the mRNA vaccines based on “neutralising antibody titres”, a surrogate endpoint. However, the product labels don’t indicate that the surrogate endpoint has not been validated or that it does not accurately reflect the degree of clinical benefit—crucial facts that the CAALM petitioners point out, and that the FDA has acknowledged elsewhere.
Updates on safety
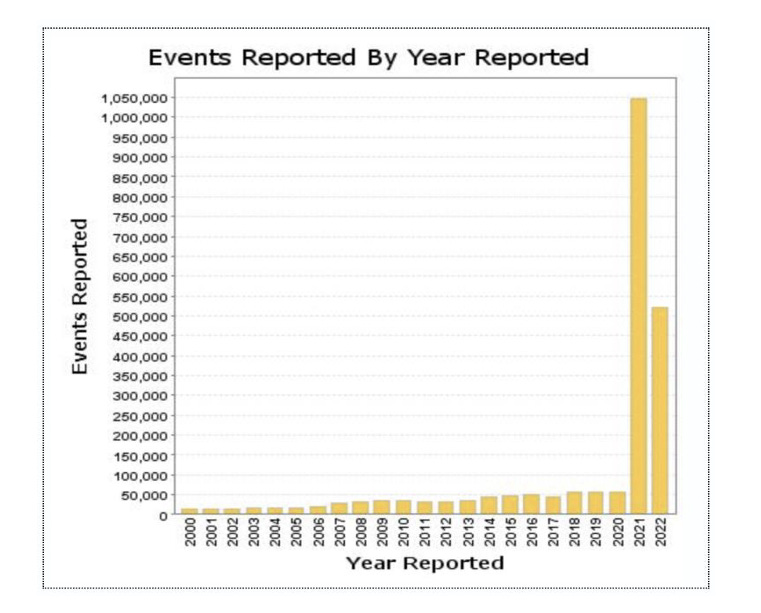
The petition highlights the dramatic increase in reports to the FDA’s Vaccine Adverse Event Report System (VAERS) since the roll out of the covid-19 vaccines.
In fact, the total number of adverse event reports between 2021 and 2022, far surpass the combined number of adverse event reports in the preceding decade.
Notably, many serious adverse events detected in post-marketing surveillance have not been accurately reflected on the vaccine labels.
Pulmonary embolism for example, is a potential serious adverse event detected by the FDA’s own post-marketing study. The finding was made between February and April 2021, and as yet, has not been added to Pfizer’s product label.
Sudden cardiac death is another adverse event linked to the mRNA vaccines based on autopsy studies from Germany and the US.
Neuropathic and autoimmune disorders have also been omitted.
Wastila laments the lack of information on the label regarding the potential harm to pregnant and breastfeeding women, or how the injection could impact fertility.
“Pregnant women are having the vaccine and not told the mRNA can enter the breast milk, or that the injection can lead to menstrual irregularities. Also, men are not told it has been shown to temporarily decrease sperm concentration,” says Wastila.
The labelling doesn’t provide accurate and updated information on the frequency of myocarditis or pericarditis either. The petitioners requested that the labels contain a range of rates that have been reported in the literature, and should stratify estimates by risk factors (notably, age and sex).
Currently, the label says “Information is not yet available about potential long-term sequelae” of myocarditis but a study by the CDC has since found that 50% (178/357) of adolescents with post-vaccine myocarditis had >1 symptom lasting 90 days from onset.
“Many colleges require all young people to get vaccinated and boosted, but few know about subclinical myocarditis” says Wastila.
“I think there could be a lot of young people walking around with no clue that they could be ticking time bombs, that they could have heart damage without symptoms, and no-one is getting them checked because no one is telling them to,” she adds.
The reaction to petition
Overall, the reaction to the petition from notable academics and scholars has been positive.
Jerome Hoffman, professor of medicine emeritus at UCLA School of Medicine, is not a signatory on the petition, but supports most of the individual points raised by the CAALM petitioners.
“Of course the FDA should be honest and include all relevant data (no matter how negative) — but not without putting it in context,” says Hoffman.
His primary concern is that highlighting the adverse events should not be done without also emphasising “that best available evidence strongly suggests that the covid vaccines, while certainly imperfect, are enormously beneficial on a population basis.”
Hoffman says it’s critical to strike a balance when communicating harms, in a manner that neither increases distrust of science, nor further emboldens vaccine hesitancy.
David Gortler, a pharmacist, pharmacologist and drug safety epidemiologist who worked as an FDA medical reviewer between 2007 and 2011, and senior adviser to the FDA commissioner in 2019-21, was pleased to see the submission.
“They're 100% right to bring this up. It's unprecedented in the history of the FDA, that there have been over 13 billion doses of the vaccine given worldwide and neither Pfizer nor Moderna has bothered to keep the label up to date with the latest efficacy and safety findings,” says Gortler. "It should be their number one priority."
“The product label is the ultimate authority and owner’s manual for doctors and pharmacists, to go to for the latest, most accurate information about a drug. It’s a living breathing document, that should be regularly updated with the latest epidemiological data,” he adds.
Gortler, who is now a fellow at the Ethics and Public Policy Center in Washington DC says, “The FDA can ask for a labelling update, but the truth is, the vaccine manufacturer is the one who ought to maintain updates. However, all vaccine makers have a liability shield, so they are not motivated to update anything.”
John Abramson, a physician and lecturer at Harvard Medical School, and author of Sickening: How Big Pharma Broke American Health Care, also supports the CAALM petition to update the vaccine labels to ensure people are able to make informed decisions.
“The lack of forthrightness about the underlying clinical trial data - and the lack of coverage of this issue by the mainstream media - are engendering dangerous vaccine skepticism,” says Abramson.
“When I went to get a booster from the local pharmacist, I mentioned that there are no data available. The pharmacist replied, ‘We are the data’ -- Americans deserve better,” added Abramson.
The petition was filed on January 31 and the FDA has 180 days to respond. Members of the public can add comments to the petition HERE.





Ethics are an interesting phenomena. They are an agreed set of standards or principals that a group of people see as being of value over a given period of time. They change as life evolves. I guess that the ethics that many of us see that big medicine and big pharma should adhere to are different from the set of ethics they operate by. And it seems their primary ethic is to maximise returns to their shareholders and investors, over everything else. This is a business model that first appeared in the 80's and it has never gone away.
In the Movie, "Other People's Money", Danny De Vito played Laurent Garfield and Penelope Ann Miller played Kate Sullivan.
Garfield: The game is, "You make as much money as you can for as long as you can. He who dies with the most wins. It's the American way."
Sullivan: "We need to pass a law to stop people like you."
Garfield: "Changing the law only alters the rules but the game continues. You can't stop the game."
These people are scoundrels.
Maryanne, I must not have been with you when you posted this. Wow.