A tense first day inside ACIP’s meeting on vaccine policy
CDC’s vaccine panel rejects the MMRV combination shot for kids under 4, stalls on hepatitis B, and pushes back on claims it’s gone “anti-science.”
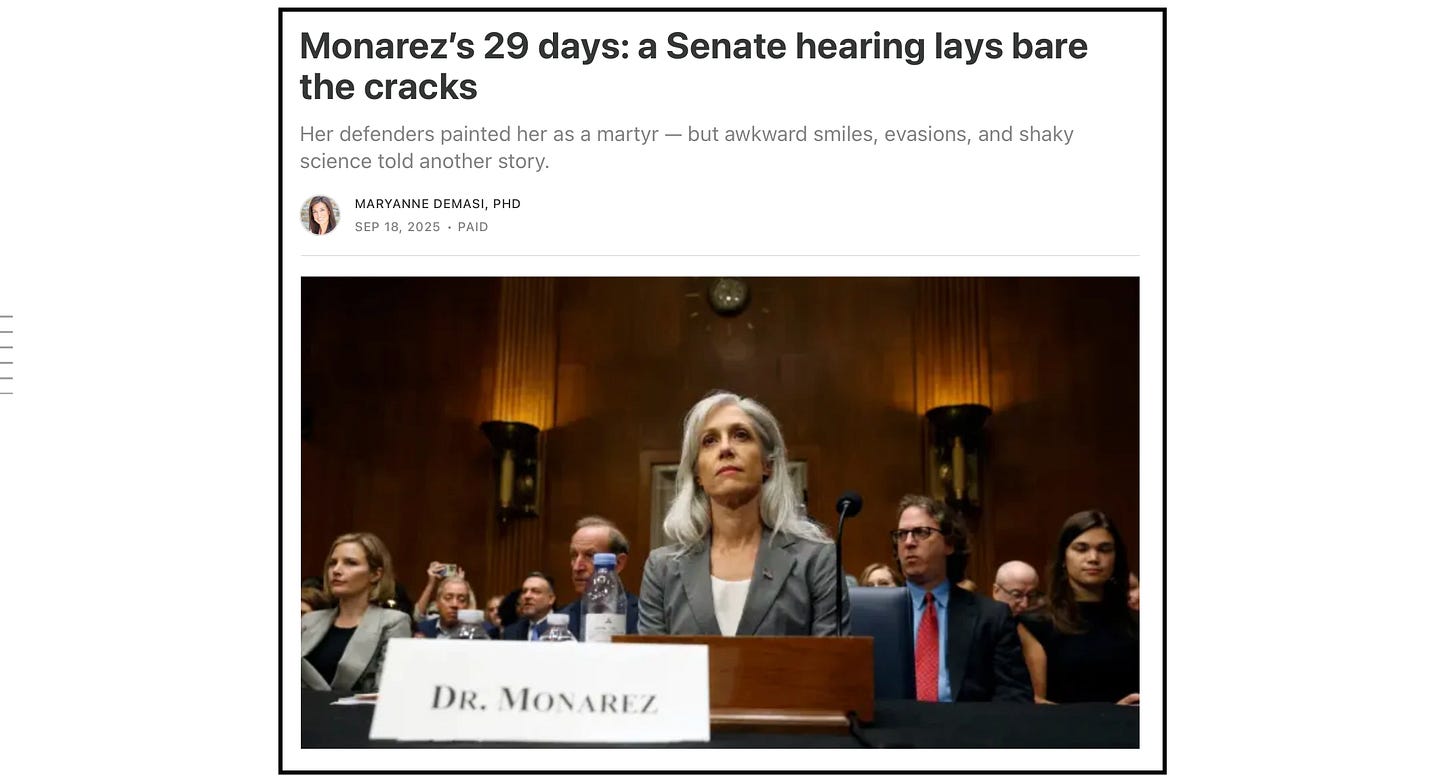
The CDC’s vaccine advisory panel opened its meeting in Atlanta under a storm of criticism.
One day earlier, former CDC director Susan Monarez told senators the committee was “unqualified” and warned that its overhaul under Health Secretary Robert F. Kennedy Jr. could pave the way for the return of polio and measles.
Her warning echoed a New York Times op-ed from nine former CDC directors, who accused the Advisory Committee on Immunization Practices (ACIP) of harbouring “dangerous and unscientific views.”
Reuters warned that Kennedy’s advisers risked exposing “many more Americans to preventable illnesses.” In The Hill, University of Utah paediatrician Andy Pavia said the panel was wasting time on “settled science.”
By the time the meeting began, the panel was already under intense scrutiny.