Are you waiting for a 'protein-based' COVID-19 vaccine?
Novavax and COVAX-19 on the horizon
By Maryanne Demasi, PhD
Early in the pandemic, most western nations favoured the rollout of ‘gene-based’ COVID-19 vaccines because they offered policymakers a speedy solution.
But the rush to bring them to market has also raised concerns about manufacturers cutting corners and fears about the unknown long-term effects of the mRNA and adenovirus vector technologies.
Moderna, for example, designed its vaccine in two days and took the unprecedented step of injecting its mRNA vaccine into humans before the completion of animal studies.
For this reason, many have vowed to put off vaccination until an alternative comes along.
Enter, ‘protein-based’ COVID-19 vaccines, such as Novavax or COVAX-19 which utilise a proven, traditional technology.
Gene versus protein-based vaccines
Whereas gene-based vaccines deliver a set of genetic instructions on how to manufacture the spike protein in the body to elicit an immune response, protein-based vaccines deliver a controlled amount of spike protein, already synthesised and purified from the lab.
It is a more traditional way of developing vaccines, and the technology has been used for decades in established vaccines such as hepatitis B, pertussis, pneumococcal and meningococcal vaccines which are administered to babies.
Protein-based vaccines require an ‘adjuvant’ to turbo boost the immune response. Novavax and COVAX-19 both use adjuvants derived from plants which have been shown to have favourable safety profiles in clinical trials.
This also mitigates concerns over the safety of adjuvants that contain aluminium or the use of polymer polyethylene glycol (PEG) in the mRNA vaccines, which can cause allergic reactions.
Adjuvants are not required for gene-based vaccines (Pfizer, Moderna, AstraZeneca, J&J) because they are so intrinsically “reactogenic”.
To synthesise the spike protein in the lab, both Novavax and COVAX-19 utilise a cell-line derived from insects (moths). This is distinct from vaccines like AstraZeneca and J&J, which use cells derived from aborted foetuses to produce the adenovirus vector and, in some countries, provides the basis for people seeking a religious exemption.
There are other advantages of protein-based vaccines; they are stable at refrigeration temperatures (2°- 8°C), allowing the use of existing vaccine supply chain channels for distribution.
Although they are not in widespread use, there are over 50 protein-based vaccines in clinical testing. Some countries such as Cuba, Taiwan and China are already using their homegrown versions, none of which are available in Australia.
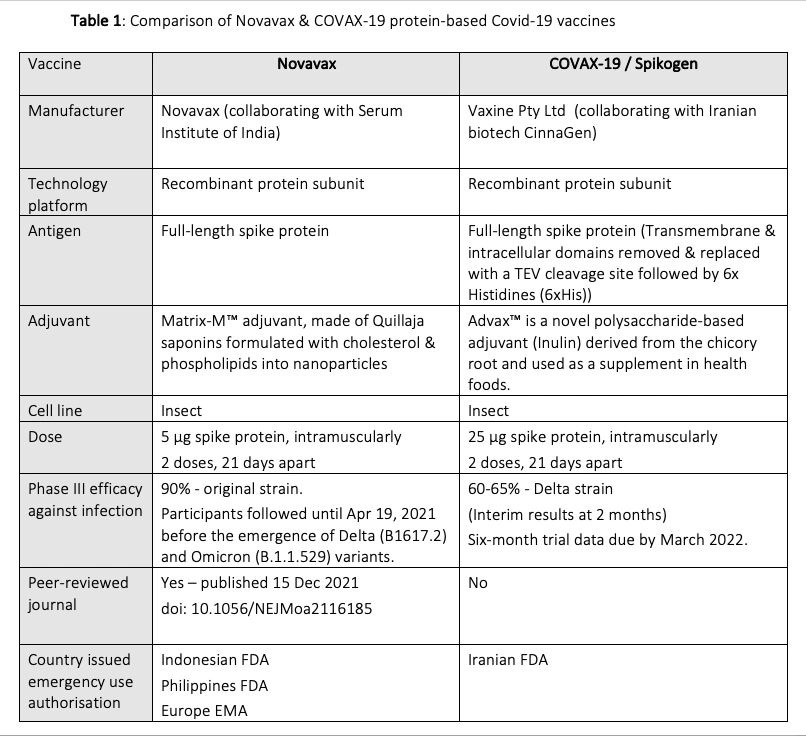
Comparison of Novavax & COVAX-19
While both vaccines use similar protein subunit technology, they differ in terms of how the spike protein is designed, purified and extracted, and the type of adjuvant used, which can translate into different safety and efficacy profiles. (See Table 1)
- Novavax
Novavax is developed by an American biotech company, based in Maryland, USA. Early in the pandemic, company boss Gregory Glenn made big promises, especially for a company that had never brought a product to market.
In 2020, Novavax was awarded US$1.6 billion from the US Government to produce up to 100 million doses as part of ‘Operation Warp Speed’ creating a lot of hype about its success.
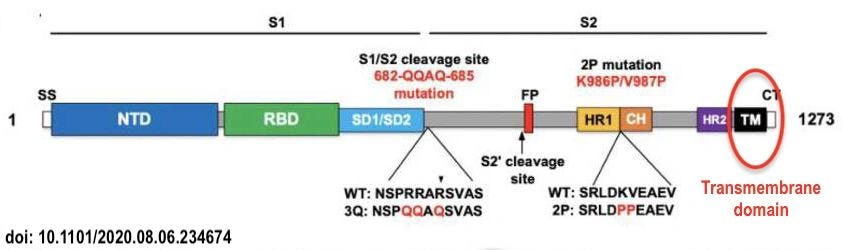
Taking a closer look at their spike protein, they decided to synthesise the “full length” of the spike protein, which included the “transmembrane domain” (circled)
Keeping the transmembrane domain in the spike protein, is a decision that, in hindsight, probably cost Novavax precious time.
The presence of the transmembrane domain causes the spike protein to “stick” to the inside of the insect cells in the lab, and it requires powerful detergents to break up the cells so that it can be extracted and purified.
Novavax has battled with a low purity and low yield product and it may explain why they struggled to attain anywhere close to the 90% purity required by drug regulators, with reports it could only achieve 70%.
To add to their woes, Novavax encountered delays in manufacturing and delivering the vaccine, indicative of the company’s inexperience in bringing a product to market.
The CEO of Novavax recently admitted to The BMJ that the company lacked the production expertise of larger corporations.
“If I can be a little bit defensive, about two years ago we were a very small company, we didn’t have manufacturing….It’s also somewhat artsy, it takes people with a lot of skills. You can’t just get people off the streets to do it,” said CEO Gregory Glenn about his company’s struggle.
Novavax struck a deal with the Serum Institute of India which has a specific plant that will manufacture the vaccine, though some say Novavax may have been better off teaming up with one of the pharmaceutical giants, just as BioNTech did with Pfizer.
So, after lengthy delays, is Novavax back on track?
Results from phase III trials published recently in the New England Journal of Medicine showed 90% efficacy against the original SARS-CoV-2 strain and other variants circulating at the time.
But because the trial was conducted in the first half of 2021 when the delta variant had not been widely established, “the vaccine efficacy against delta and other newer variants could not be established.”
Recently, Novavax announced its current vaccine could be effective against omicron, but that it would begin clinical studies on an omicron-based COVID-19 vaccine in the first quarter of 2022.
In November 2021, Indonesia became the first country in the world to grant Novavax an emergency use authorisation, followed closely by the Philippines.
In December 2021, Europe’s drug regulator (EMA) also granted conditional marketing authorisation and the WHO issued an emergency use listing to expand its bevy of coronavirus vaccines.
The Australian Government is still waiting on 51 million doses pending provisional approval by the TGA, which the drug agency says is currently being assessed.
Novavax says a submission to the US FDA is imminent, but it has broken promises before. Some are sceptical since Novavax has been trying to develop a protein-based “flu” vaccine but has failed to win regulatory approval by the FDA.
Now Novavax is angling for a combo vaccine to fight both COVID-19 and influenza – trials are currently underway in Australia, the results of which, are due later this year
- COVAX-19
Spearheaded by Professor Nikolai Petrovsky at Flinders University, COVAX-19 was developed by Vaxine Pty Ltd, an Australian company based in Adelaide, South Australia
Unlike Novavax, the company has decades of experience in developing protein-based vaccines like the one it produced for the 2003 SARS outbreak or MERS in 2012.
COVAX-19 received minimal financial support from the Australian Government, which favoured funding other candidates, including the failed University of Queensland vaccine that left trial participants testing “false positive” to HIV.
Further, the spike protein in COVAX-19 has had the transmembrane domain removed.
By removing the transmembrane domain, the spike protein is able to be excreted from the cell (doesn’t stick to membrane), so it doesn’t require detergents, resulting in high purity and high yield.
Pre-clinical testing in ferrets and mice found a potent antibody and T-cell response after vaccination. A phase I human clinical trial commenced mid-2020 in Australia and the Phase II/III trials were conducted in Iran.
According to Prof Petrovsky, the phase III clinical trial in 16,876 subjects showed the vaccine had 60-65% efficacy against infection after two months of follow up, surpassing the WHO and FDA criteria for COVID-19 vaccine approval.
It is a far cry from the >90% efficacy seen in the early trials of its competitors, but Prof Petrovsky explains that COVAX-19 trials took place under more challenging conditions when the highly infectious delta variant was dominant.
If you look at Pfizer’s efficacy against delta, it reportedly dropped to 39% and would have failed to meet the criteria for FDA approval if it had been trialled in late 2021 against the newer variants.
As yet, there are no data on the effectiveness of COVAX-19 against the omicron variant, but trials are underway.
In Oct 2021, the Iranian Food and Drug Administration (IFDA) granted an emergency use authorisation for COVAX-19 vaccine (also known as Spikogen), manufactured by Iranian biotech company CinnaGen.
Without the luxury of the Australian Government’s pre-purchase orders, Prof Petrovsky's team turned to the public for financial support and established a GoFundMe page to help raise funds.
The money will cover the cost of submitting a dossier to the TGA (AUD$300,000), which is expected to be filed this month, as well as an ongoing phase IV long-term safety and effectiveness trial.
Over two million doses of COVAX-19 have been administered throughout Iran, and according to Prof Petrovsky, “pharmacovigilance data indicate there have been no reports of anaphylaxis, myocarditis or blood clotting.”
Results from phase III trials have not been published in peer-reviewed journals which has drawn criticism by academics.
“We don’t know this vaccine is safe and effective yet. It might prove to be in the future, but we have insufficient [publicly available] evidence at this point in time,” said infectious diseases expert Associate Professor Paul Griffin to The Guardian.
Prof Petrovsky defends the company’s decision to prioritise the dossier for regulatory approval and cites a lack of resources for the delay.
“The regulatory approval and submissions have priority right now. Obviously, we'd like to get the information publicly available and published, but we have a small team with no resources, and no support. Unlike Pfizer, we can’t just pay a company to ghost-write papers for us,” said Prof Petrovsky.
Interestingly, his team explored a variety of platform technologies early in the pandemic and decided against advancing with their “mRNA” vaccine.
“One reason was we just didn’t believe mRNA was ready for the primetime. Vaccine experts just thought it would never get regulatory approval because there was no long-term safety data on it. We thought that would be insanity,” said Prof Petrovsky.
“The other reason was that we saw the early phase I data and it was dreadful. The reactogenicity was horrific - 15 to 20% of people getting high fevers, and people being sick for up to a week,” says Prof Petrovsky.
“As a vaccine developer, that would have killed the program then and there, if it had not been in the context of Covid, a Trump administration, and the political pressure that came with that,” he added.
It may take several months before Australia and other countries see these vaccines being administered, but many have welcomed the new crop of protein-based COVID-19 vaccines because it will expand people’s choices.
Disclosure: Author currently registered for Phase IV COVAX-19 clinical trial