Drug regulators are hiding RNA stability data
Remember when there was panic in 2020 because Pfizer’s mRNA COVID-19 vaccine couldn’t be transported across the country unless it was stored at ultra-freezing temperatures?
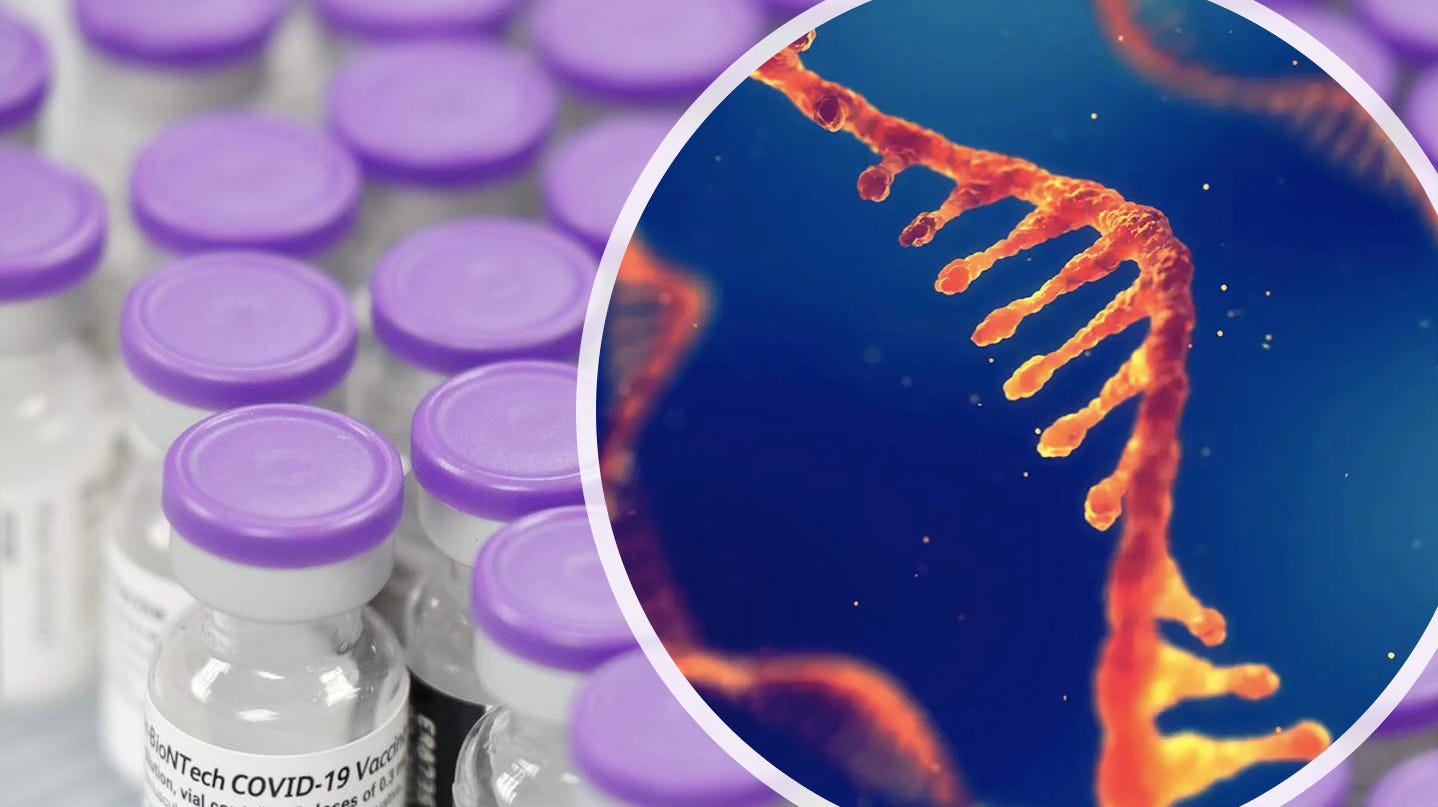
Pfizer said the mRNA in the vaccine, which coded for the spike protein, was unstable and would decay if the unopened vials were not kept at -70ºC.
So, when the FDA granted authorisation in December 2020, it specified the vaccine had to be stored between -80ºC and -60ºC, requiring special ultra-cold freezers, which proved challenging to areas with limited resources.
But by February 2021, Pfizer had apparently solved the problem.
It submitted new “RNA stability data” to the FDA demonstrating the vaccine could be stored in conventional freezers (-20ºC) and no longer required ultra-cold freezers.
The FDA approved the change swiftly.
Two months later, Australia’s Therapeutic Goods Administration (TGA) also approved Pfizer’s application, allowing unopened vials to be stored at -20ºC for up to 2 weeks.
Storage temperature wasn’t the only change. Drug regulators also approved extensions to the vaccines’ expiry dates.
Various batches of Pfizer’s vaccine, for example, had their expiry dates extended by one year (FDA) or 6 months (TGA).
But given the sensitivity of RNA to changes in temperature and storage duration, what stability data did the regulators rely on to green-light these decisions?