Experts weigh in after suing FDA for access to Pfizer trial data
By Maryanne Demasi, PhD
In Dec 2020, the US drug regulator (FDA) granted emergency use authorisation to Pfizer’s Covid-19 mRNA vaccine with limited data from phase III trials.
Amid the urgency of the pandemic, the review of the trial data was conducted faster than usual. What would normally take an average of 10 months to review, only took the FDA 108 days.
Immediately, it raised doubts about the speed with which the agency made its decision.
Fast forward to Aug 2021 and the FDA granted full approval to Pfizer’s (Comirnaty) mRNA vaccine, without releasing the full data set to the public for independent scrutiny.
Experts became concerned that all the publicly available information on a fully licensed product, was limited to journal articles, press releases, and assessments by drug regulators – all of which are subject to conflicts of interest and bias.
Scientists rally for transparency
A group of over 80 public health officers and medical researchers formed an alliance to obtain and disseminate the data upon which the FDA made its decision to license Pfizer’s vaccine.
The non-profit group, called Public Health and Medical Professionals for Transparency (PHMPT) filed a lawsuit in the U.S. District Court, Fort Worth, Texas in September 2021. US-based physician, Dr Aaron Kheriaty, is one of the members leading the charge.
“A group of us were concerned about the trial design, the shortened duration of the clinical trial, and the patchwork system that was in place for the post-marketing surveillance of adverse events,” said Dr Kheriaty.
For example, Pfizer was allowed to terminate its control group after only two months.
“The placebo group was basically eliminated because the vaccine was offered to everyone who had the placebo, so they failed to maintain a control group,” said Dr Kheriaty.
The Freedom of Information Act (FOIA) lawsuit stipulated that, under federal law, the data and information in the documents filed with the FDA should be available for public disclosure unless extraordinary circumstances are shown.
The purpose was to ensure government transparency and accountability.
Prof Tom Jefferson is another member of PHMPT and of the WHO’s Covid-19 Infection Prevention and Control Research Working Group. He says the importance of an independent review of the scientific data cannot be overstated.
“Censorship and lack of transparency have always been the enemies of progress. In the case of Covid-19 vaccines, the importance of transparency in heightened by the mass administration to healthy populations and their unknown long-term effects,” said Prof Jefferson.
“Given the insufficient and hurried testing and the culture of secrecy, it is arguable whether any informed consent is valid, prior to making public all of the documents the FDA has in its possession,” he added.
Pfizer’s vaccine has been the subject of vigorous debate, including claims of under-reporting adverse events, falsification of data, and a lack of efficacy.
FDA asks to delay document release
While the FDA says it’s committed to transparency, the agency proposes to drip feed Pfizer’s documents to the public over several decades.
The Department of Justice (DOJ) lawyers representing the FDA asked the federal judge to allow them 75 years to process the FOIA request, which takes the end date for the final release of documents to 2096.
They argued that releasing 451,000 pages of documents immediately would be too burdensome because the agency was insufficiently staffed and that it would only be able to release just 500 pages per month to allow for redactions of exempt material such as trade secrets.
“The idea that the FDA with 18,000 employees and $6.5 billion in funding cannot produce the documents more expeditiously is absurd,” says Aaron Siri, US attorney acting on behalf of PHMPT, the plaintiff.
“It is dystopian for the government to give Pfizer billions, mandate Americans to take its product, prohibit Americans from suing for harm, but yet refuse to let Americans see the data underlying its licensure.”
“The FDA has not disputed that it should produce these documents” said Mr Siri, “rather, it proposes doing so at a rate so slow that the documents will not be fully produced until almost all of the scientists, attorneys, and most of the Americans that received Pfizer’s product, will have died of old age.”
In rebuttal, the FDA said it is unreasonable to expect them to meet the demands because it only has 10 employees processing FOIA requests. Mr Siri says that’s no excuse.
“There are numerous instances of other agencies, when dealing with a production that is eligible for expedited processing, that have transferred staff, or hired more staff, in order to promptly comply with their statutory obligations,” says Mr Siri.
Indeed, in the DOJ’s response to the court, it conceded that since 2018, the FDA has responded to federal subpoenas, with quick turnarounds for productions that had required hundreds of thousands of pages each.
What did the initial release of documents show?
A batch of Pfizer’s documents has already been released by the FDA.
Dr Kheriaty said there was a lot of hype in the media over the meaning of the documents, but there is still a lot of missing information and therefore, is cautious not to over-interpret the data so far.
For example, there were claims that the documents showed 1223 people had died from the vaccine, within the first 90 days of the vaccine rollout, but the reality is more nuanced.
“Basically, we just have raw numbers. If you look at that document, they redacted information about how many Pfizer doses had been shipped out. So, if we don't know how many total doses were given, we cannot establish what percentage of people who got the vaccine, may have had those adverse events,” explained Dr Kheriaty.
That said, the number of ‘reported’ deaths received by Pfizer in the early stages of the vaccine rollout did strike Dr Kheriaty as 'high.'
“It’s a huge spike there that should be taken as a significant safety signal”, said Dr Kheriaty.
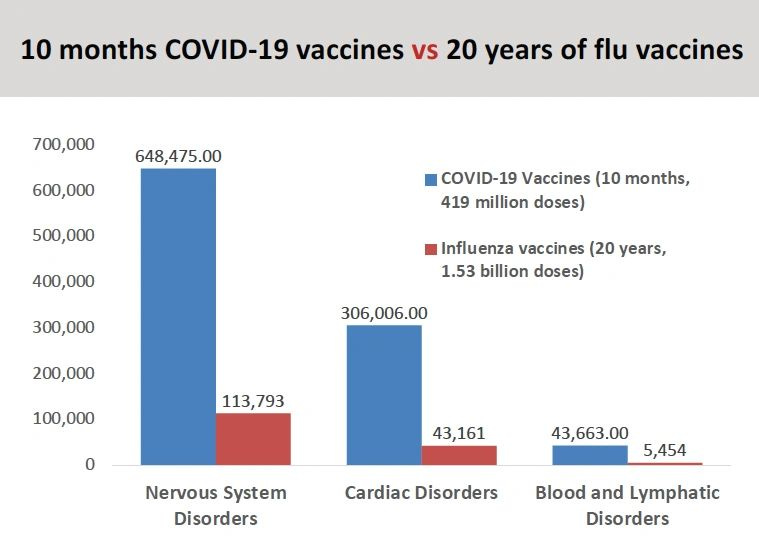
“If you look at vaccine adverse events that have been reported for the Covid vaccines compared to the flu vaccine over the last 20 years, we've seen orders of magnitude larger numbers of adverse events, including deaths, reported.” (see graph)
Further, he pointed to the appendix in one of the Pfizer documents (page 30) that listed nine pages of reported adverse events.
While causation cannot be inferred, Dr Kheriaty suggested that there are some concerning signals in the list.
“I'm seeing a lot of neurological issues and I'm seeing a lot of autoimmune issues, “he said.
“If you look at the fact sheet given to people receiving the Pfizer vaccine, it lists something like 21 potential side effects from the trial, most of them benign. In contrast, the adverse events reported in those first 90 days of post surveillance are very concerning.”
Until the entirety of the data are released, a definitive assessment cannot be made.
Prof Jefferson shares a similar view.
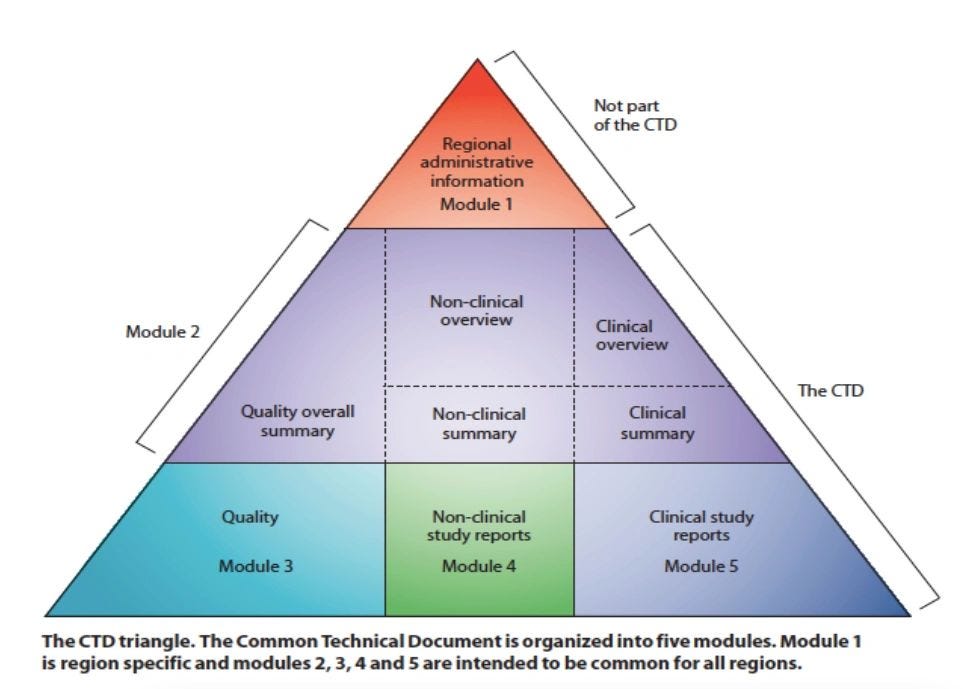
The Pfizer documents submitted to the FDA are likely to follow an international standard structure - called the Common Technical Document (CTD), which consist of five modules.
“Partial, incomplete, or batch release of parts of the CTD impede assessment of the application in a coherent way and may lead to errors in the interpretation of its content,” says Prof Jefferson.
Releasing the modules out of sequence - which is what the FDA is doing now – will adulterate the analysis.
“Missing even a single dataset could corrupt any analysis by scientists seeking to conduct a proper review of Pfizer’s data, that is why it should all be released immediately,” he said.
What now?
While the FDA has already released a batch of Pfizer’s documents, Mr Siri says the judge is yet to decide on how the FDA should proceed.
“There has been no decision issued in this case yet and any documents produced to date have been produced by the FDA apparently in the hopes of softening any decision the Court may render,” he says.
A final ruling by the judge on the time frame that the FDA must release its documents could be determined within weeks unless the hearing is adjourned.