Pfizer, Moderna win authorisation for 6 months & up
By Maryanne Demasi, PhD
Last week, the US FDA convened its expert panel - the Vaccines and Related Biological Products Advisory Committee (VRBPAC) - to discuss the authorisation of Moderna and Pfizer covid-19 mRNA vaccines for infants and pre-schoolers.
Lengthy briefing documents (250 pages) were sent to the VRBPAC members with only days to consider the content.
It was a highly anticipated meeting, but the outcome seemed inevitable after the FDA’s top official Peter Marks had already told a congressional committee that the agency would not expect the same 50% threshold for vaccine efficacy against covid-19 infections, that was expected of the adult trials.
The FDA’s VRPBAC voted unanimously (21-0) to authorise emergency use of:
Pfizer’s three-dose vaccine (10µg each) for children 6 months to <5 yrs
Moderna’s two-dose vaccine (25µg each) for children 6 months to <6 yrs
The decision was later endorsed by the US Centres for Disease Control and administration to 20 million eligible children in the US is expected to begin this week.
This decision is at odds with other countries like Sweden where officials do not recommend vaccinating children 5-11yrs, and many other European countries like Denmark, Norway, Finland, France, and Iceland which have halted the use of Moderna’s vaccine in adolescents and young adults.
Lower burden of proof
Emergency use authorisation via the FDA’s expedited pathway requires a lower burden of proof compared to the more traditional pathways of approval.
Instead of evaluating the reduction of important outcomes such as covid-19 hospitalisations, Pfizer and Moderna relied upon a method called “immunobridging.”
Put simply, the neutralising antibody levels in kids are compared to adults from another trial, and protection against infection is “inferred”.
The advantage of this approach is that the vaccine manufacturers can run shorter, less rigorous trials with fewer subjects, saving them millions of dollars.
This inferior evidentiary threshold of immunobridging has been acknowledged by the FDA. Peter Marks, who heads the FDA’s Center for Biologics Evaluation and Research (CBER) recently said, “We’re using poor person’s correlates of protection with antibody levels.”
In fact, the FDA’s own website says that “antibody tests should not be used to evaluate a person’s level of immunity or protection from covid-19.”
Poor efficacy
Moderna and Pfizer recruited about 3-4,000 subjects for each of their trials, but antibody testing was only performed on a subset of participants – no explanation was given for why much of the data were excluded.
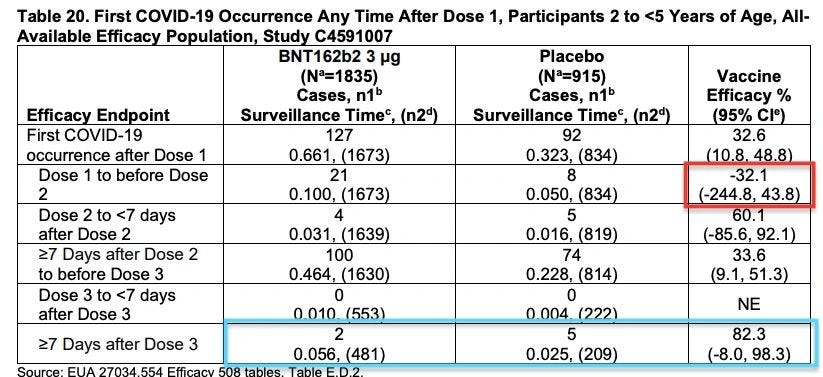
Pfizer’s vaccine efficacy between dose 1 and 2 plummeted into the negative, i.e. vaccinated kids aged 6 months to <5 yrs, were more likely – albeit not statistically significantly - to become infected with covid-19, than unvaccinated kids after their first dose (red box).
Pfizer claimed that vaccine was 75 to 82% efficacious after dose 3 (blue box), but only if you ignore all the data that preceded it.
The problem is that it was only based on a total of 10 cases and the confidence intervals were so wide (varied from -370% to 99%), that no sensible conclusions could be drawn from the data. In fact, Marty Makary from Johns Hopkins University commented “those are the largest confidence intervals I’ve even seen in my career. Basically, zero confidence in the results.”
VRBPAC member Paul Offit, director of Children’s Hospital of Philadelphia’s Vaccine Education Centre, was also concerned by Pfizer’s “surprisingly poor” vaccine efficacy and said that he expected this age group would probably need a "fourth dose."
Moderna’s vaccine showed between 37% and 51% efficacy in infants aged 6 months to <6 years, peaking at 42 days after the first jab. Unlike Pfizer’s trial which saddled the omicron surge, Moderna’s trial was completed in 2021, and could not confirm if its vaccine would have any lasting effect against omicron.
What is clear for both vaccines, is that the antibody levels are likely to wane quickly. Both vaccines were designed to target the original Wuhan strain of the SARS-CoV-2 virus and the omicron variant is better than previous variants at evading vaccine-induced immunity.
Natural immunity
Another deeply problematic issue regarding the Moderna and Pfizer trials is that only 7-12 % of the children had pre-exposure to the virus. This has no relevance to the real-world population.
CDC data show the vast majority of children and adolescents (over 75%) have already been exposed to the virus and have acquired natural immunity, making the trial results not generalisable.
Experts hoped that the vaccine would reduce the risk of infection, and re-infection, with covid-19. But in the Pfizer trial, a total of 12 kids were re-infected with covid-19 during the trial; 11 of them were vaccinated, one was unvaccinated.
This appears to corroborate real world data from countries like Scotland where there are higher rates of infection among those who are the most vaccinated, raising previous concerns that the vaccines might be suppressing a person’s immune responses to infections.
Harms
Only hours after the CDC rubber stamped the FDA’s decision, Director Rochelle Walensky beamed with confidence that the vaccines could be safely administered to US children under 5 years.
However, there were not enough participants in either trial to determine if there are any serious but rare harms caused by the vaccine. The follow up on trial participants was also too brief (~2months).
In the Moderna trial, there were significantly more “off-target” events of croup, respiratory syncytial virus (RSV), and pneumonia in vaccinated infants (6-23 months) – in fact, it has been documented in many studies that vaccination with a non-live vaccine increases the risk of other infections – but the FDA dismissed the findings saying that there was no biological mechanism for these events and therefore, unlikely to be causal.
The risk of myocarditis and pericarditis in young adolescents was raised as a concern, but these adverse events were not detected in kids 5 and under.
The FDA conceded that there was not information available about the potential long-term harms in this age group, and that they did not know whether the vaccine might be causing “subclinical” myocarditis (without symptoms), especially in babies who cannot verbalise their symptoms.
One interesting observation was that adverse events in the placebo groups were very high – or at least much higher than expected for a recipient of a ‘saline’ placebo injection. For example, Pfizer reported that after the first dose, 61% of infants reported a systemic adverse event compared to 58% in the placebo group.
Sceptics have suggested that, instead of saline, the placebo injection must have contained the carrier (empty lipid nanoparticles) but these claims have not been substantiated.
Another complication of running a trial in this age group is that many are still receiving all their other routine childhood injections. When this was raised as a concern by one of the VRBPAC members, it was not clear how anyone would tease out the impacts of the mRNA vaccine from the concomitant vaccines.
Both Moderna and Pfizer promised to continue to surveil the trial subjects for adverse events for 6 months but if significant safety signals emerge by that time, thousands, if not millions of infants would already have been vaccinated.
Allaying fears
The CDC claimed that 442 kids in this age group had died from covid-19 during the pandemic. Parents have pleaded with the FDA to authorise the vaccines – one said that her 3-year-old had never been inside a restaurant, amusement park or grocery store, to avoid catching covid-19.
VRBPAC member Cody Meissner stressed that risk had to be put into perspective. “We've heard several times that there were approximately 442 [covid] deaths, so far in the pandemic among children less than 5yrs old. So that means about 220 deaths a year. If you look at the number of people who are struck by lightning in the United States in a year, it's 270. So, we're talking about a very rare event….I think that has to be communicated clearly to parents so that they can participate in the decision about vaccinating a child in this age group.”
In the end, the VRBPAC members explained that the vote was about offering parents a choice. But given the absence of a genuine public health emergency in this age group, many say the FDA should have insisted on better evidence, longer trials, and more meaningful clinical outcomes.