Secrecy fuels COVID-19 vaccine hesitancy
By Maryanne Demasi, PhD
Fed up with lingering vaccine resistance, US President Joe Biden recently delivered a sharp rebuke to unvaccinated people, saying that “they get sick and fill up our hospitals,” taking beds away from others who need them.
The sentiment is echoed by some in the medical fraternity.
An Australian doctor has banned unvaccinated patients from his Tasmanian practice. And an Alabama doctor recently announced that he would refuse to treat patients if they hadn't been vaccinated against COVID-19.
“I’m running out of compassion for the unvaccinated,” another doctor commented in the LA Times when an unvaccinated COVID-19 patient wound up in hospital.
“I struggled to find sympathy” she said after her patient was worried about taking an experimental vaccine that was not FDA-approved.
The doctor told her patient, “I can pretty much guarantee we would have never met had you gotten vaccinated, because you would have never been hospitalised.”
While the doctor's comment is spurious (fully vaccinated people are still being hospitalised), what is clear, is that empathy for “vaccine-hesitancy” is waning fast.
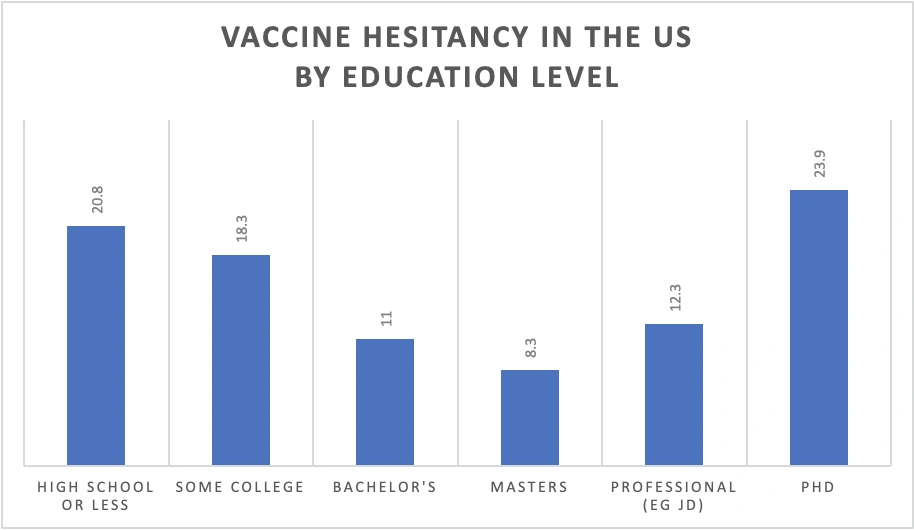
It is easy to disregard them all as ‘anti-vaxxers’ with poor vaccine literacy, but an online survey of over 5 million US citizens was reported to show that the people with the highest level of education (PhDs), were the most vaccine-hesitant.
While more people are fronting up to get the jab, vaccine hesitancy still lingers and the rates of fully vaccinated people in countries like Australia and the US remain inadequate.
So, what is impacting vaccine hesitancy?
Transparency concerns
The fact is, COVID-19 vaccines were developed at warp speed. Full transparency of the clinical trial data is a powerful antidote to vaccine hesitancy. But transparency took a back seat this week.
The US Food and Drug Administration (FDA) granted full approval to the Pfizer vaccine without a public discussion of the submitted data, the fastest approval in the agency’s history.
In doing so, the FDA reneged on its promise to “use an advisory committee composed of independent experts to ensure deliberations about authorisation or licensure are transparent for the public”.
The FDA told the BMJ it had already held public meetings about the emergency use authorisation and therefore, did “not believe a meeting [was] needed” for the full approval.
This had transparency advocates reeling.
“These public meetings are imperative in building trust and confidence especially when the vaccines came to market at lightning speed under emergency use authorisation,” said drug safety advocate Kim Witczak to the BMJ.
“The public deserves a transparent process, especially as the call for boosters and mandates are rapidly increasing. These meetings offer a platform where questions can be raised, problems tackled, and data scrutinised in advance of an approval,” added Witczak.
Why the rush to fully license the Pfizer vaccine with only six months of data? Some argue it was political pressure. The FDA’s decision will give cover to those institutions making the vaccine mandatory, and it will also make it easier for physicians to prescribe booster shots.
One of the objections to mandatory vaccination has been that the vaccines are still “investigational”, but this is no longer valid for the Pfizer vaccine.
Pfizer chief executive Albert Bourla said in a statement. “I am hopeful this approval will help increase confidence in our vaccine.”
What about the other US vaccines that only have emergency use authorisation? Moderna filed for full approval 1 June 2021, and Johnson & Johnson says it plans to apply for approval later this year.
If national drug regulators appear to be making crucial decisions behind closed doors, it will create a fertile ground for mistrust.
Decisions without data
Vaccine manufacturers are expected to make their data rapidly available. In a pandemic, decisions need to be made quickly, especially when administering vaccines to millions of people, with new platform technology (mRNA vaccines) before phase 3 trials have been completed.
In May 2021 however, a report by Transparency International (TI) identified 86 registered clinical trials across 20 covid vaccines, and only 12% of the trials had publicly available protocols.
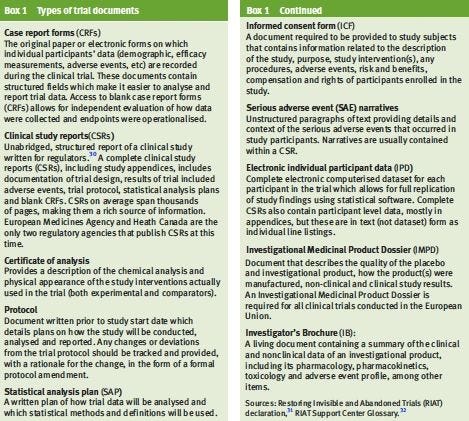
Protocols and other regulatory documents (see Box1), contain important information such as trial design, objectives, methodology, and statistical analysis plans, that allow researchers to independently evaluate how the data were collected.
In addition, the TI report showed that clinical study reports were only made available in two of the nine countries that are developing vaccines.
Similarly, researchers published an analysis in BMJ-Evidence Based Medicine, expressing concern about the “inadequate availability” of COVID-19 vaccine trial documents and data.
They found that protocols and informed consent forms are not available for trials involving special populations such as children and pregnant women.
And despite vaccine roll-out to millions of people, electronic individual participant data (IPD) are not available for most of the vaccine trials, meaning the vaccine data cannot be independently verified.
“It is a scientific, moral and ethical imperative that access to complete trial data of these global public health interventions is urgently granted to patients, researchers and other key stakeholders,” wrote the researchers.
Cutting corners
In a feature published in The BMJ, A/Prof Peter Doshi explains that despite protestations by government officials that “no corners were cut” in the development of COVID-19 vaccines, there may have been some compromises.
According to A/Prof Doshi, one type of study which tracks the distribution of a vaccine once injected in the body (biodistribution study), was not provided using any of the three vaccines currently authorised in the US.
These biodistribution studies are standard in drug safety testing but not usually required for vaccines, unless “new delivery systems are employed or when the vaccine contains novel adjuvants or excipients,” states the European Medicines Agency.
For vaccines such as J&J or AstraZeneca, they deploy adenovirus vectors, the same technology used in the Ebola vaccine, so we’ve had previous experience with these viral delivery systems.
The mRNA vaccines, however, are implementing new technology and according to The BMJ, the manufacturers Pfizer and Moderna, nor the FDA, would say whether new biodistribution studies would be required prior to licensure.
Vaccine Indemnity
It is now widely known that many governments around the world have struck deals with various vaccine manufacturers to indemnify them from any claim to financial damages in the event of vaccine-related injuries.
Therefore, someone who suffers an adverse event after being vaccinated can file a claim against the manufacturer, and, if successful, the government would pay the compensation.
The deal ensures that vaccine developers are not dissuaded from deploying vaccines out of fear of litigation, and it also encourages people to get the jab.
The Australian government has gone further and recently tweaked its indemnity deal to also protect health practitioners.
"The government will step up to the plate and take on that extra indemnity” said the Australian Medical Association's national President Omar Khorshid to the ABC following the announcement.
"That's going to reduce the chances that GPs, or other practitioners like nurses or pharmacists... get caught up in legal action."
Recently, it was revealed by The Bureau that Pfizer wanted additional protections in their indemnity deal, asking countries like Brazil and Argentina to put up sovereign assets, which might include embassy buildings or military bases, as collateral against the cost of future legal cases.
Most of these contracts cannot be scrutinised, creating an information vacuum that, in the end, leaves people feeling more hesitant, not less.
Censorship breeds mistrust
Outside of the vocal minority of anti-vaxxers who have ideological or theoretical objections to vaccines in general, there is a significant cohort of health professionals afraid to express their scientific concerns about COVID-19 vaccines for fear of being ridiculed by their colleagues or reprimanded by their institutions.
In Australia, the national medical regulator (AHPRA) has issued a position statement clearly stating that doctors will face disciplinary action if they undermine the COVID-19 vaccination campaign. For example, any anti-vaccination material on social media "may result in prosecution by AHPRA” and “practitioners must be careful not to discourage their patient or client from seeking vaccination.”
The medical regulator justifies its position saying that doctors need to uphold and promote good public health advice, but some argue that suppressing a doctor’s ability to publicly express hesitancy about the newly minted vaccines, is unreasonable.
In a review of a new book titled, Vaccine Hesitancy: Public Trust, Expertise and the War on Science, Harvard-trained Dr Adam Urato agrees with the author Maya Goldenberg, who says that instead of blaming “public ignorance” for vaccine hesitancy, corporate influence of the medical establishment is largely to blame.
“Simply put, the public has lost trust in medicine because medicine is now seen to have been corrupted by corporate cash”, writes Dr Urato.
“The medical establishment has, in many respects, been taken over by the drug industry. This has led to medical policies and practices that put corporate profits above the public interest. And the public has lost trust in this rigged system.”