The Great Debate: Port Hedland vs The Premier
I addressed the TGA's malfeasance in managing concerns regarding the COVID-19 mRNA vaccines.
On November 29, 2024, I took part in The Great Debate: Port Hedland vs The Premier, which was hosted at Perth's Convention and Exhibition Centre in Western Australia. This event brought together a panel of distinguished speakers, including politicians, scientists, and legal experts, to discuss and critique the Australian government's approach to the safety concerns surrounding COVID-19 vaccines, advocating for increased transparency and better public health policy.
My role at the event was to shed light on what I believe to be malfeasance by the Therapeutic Goods Administration (TGA) in dealing with the ongoing issues related to the excessive levels of plasmid DNA fragments found in Pfizer and Moderna's mRNA vaccines. This topic has become a significant point of contention, highlighting the need for rigorous oversight and accountability in vaccine safety protocols.
Despite an invitation, Western Australian Premier Roger Cook, chose not to attend, signalling a lack of accountability and engagement on a critical public health issue.
The entire event with all the speakers can be watched on Rumble, but I provide my slides and notes below:
Drug regulators consider residual DNA to be a “process related impurity.” Normally, the plasmid DNA, which is used to manufacture the vaccine, is digested by enzymes and removed from the final product.
Drug regulators have a permissible limit for residual DNA in biological products like vaccines, but that limit has increased significantly over time. In 1985, the US FDA set an upper limit of 10 picograms per dose. Today, that limit has increased 1000-fold to 10 nanogram per dose.
According to the TGA’s website, its role is to safeguard and enhance the health of Australians through its regulation of therapeutic goods.
I put the TGA under the microscope and compared it to 5 other major international drug regulators. The investigation was published in The BMJ in 2022.
Compared to other drug regulators, the TGA has the highest percentage (96%) of its operating budget that comes from the drug industry - the very industry its meant to regulate.
94% of drug applications submitted to the TGA are approved and 20% of drugs are approved via expedited pathways.
These expedited pathways have a lower burden of proof for safety - and this is the pathway used to grant “provisional approval” of the covid-19 mRNA vaccines in 2021.
We’ve heard about the independent studies that found excessive levels of residual DNA in the mRNA vaccines. In particular, virologist David Speicher reported levels in Australian vials that exceeded the limit by 7 to 145 fold.
But in a statement released on October 18, the TGA said these studies were “misinformation,” and that they were not robust or reliable.
In that statement, the TGA also made several patently false and misleading claims, which I will address tonight.
The TGA said that all covid vaccines were “rigorously assessed.” However, as I mentioned, the vaccines were rushed through an expedited approval process with no data on medium or long-term safety, meaning the the drug sponsor and regulator were supposed to closely monitor the pharmacovigilance data for harms.
The TGA also did not demand any carcinogenicity or genotoxicity studies from the vaccine manufacturers, prior to rolling out these vaccines to the public.
And finally, Pfizer’s product used in the clinical trials, was not the same product that was injected into people. PROCESS 1, a stringent methods, was used to make the product tested in the pivotal clinical trials. But when it came to upscaling the production of the vaccine to make billions of doses, Pfizer switched to PROCESS 2, which is how the problem with excessive residual DNA arose.
So, the TGA did not “rigorously” assess the products for safety.
The TGA had concerns about the integrity of the samples tested and said that some had past their used by dates, some had already been opened and therefore, questioned the validity of the findings.
However, most of the tested vials were sealed and not tampered with.
Some were expired and some were not, but I will explain in the next slide why this doesn’t matter.
The point here is that Prof Philip Buckhaults actually sourced unopened vials that were not expired, and still found residual DNA.
And finally, ‘reproducibility’ is important in science. We now have at least five independent studies, from different laboratories, all arriving at the same conclusion. That means, these findings have been adequately validated.
The TGA said that the samples were not kept in ‘cold chain’ and did not have temperature loggers. This might be important if you’re testing mRNA stability because RNA is an unstable molecule.
However, DNA is very stable. Every one knows that cold cases can be cracked decades down the track because forensic experts are able to extract DNA from dried blood - that’s how stable DNA is.
The TGA said that using the fluorometry overestimates levels of DNA - and that is true. But David Speicher was very clear about adding another step to this methods to mitigate the problem. He used “RNAse” enzymes to digest the RNA, leaving him with a much cleaner sample of DNA.
Moreover, this was not the only method used to assess residual DNA. Speicher, Buckhaults and McKernan have all used quantitative PCR - which happens to be the same method used by the TGA.
This method, according to Kevin McKernan, underestimates the level of residual DNA by up to 100-fold.
The TGA said that no regulator has established a causal link between the vaccines and any type of cancer. It’s true that the original trials were too short in duration to see rises in cancer and that’s why pharmacovigilance is so important.
So, we asked the TGA for its data on cancer incidence in people who received the mRNA vaccines and the FOI request (5275) came back saying that the documents “do not exist.” Hence, the TGA is not even looking for a link and therefore, can maintain plausible deniability.
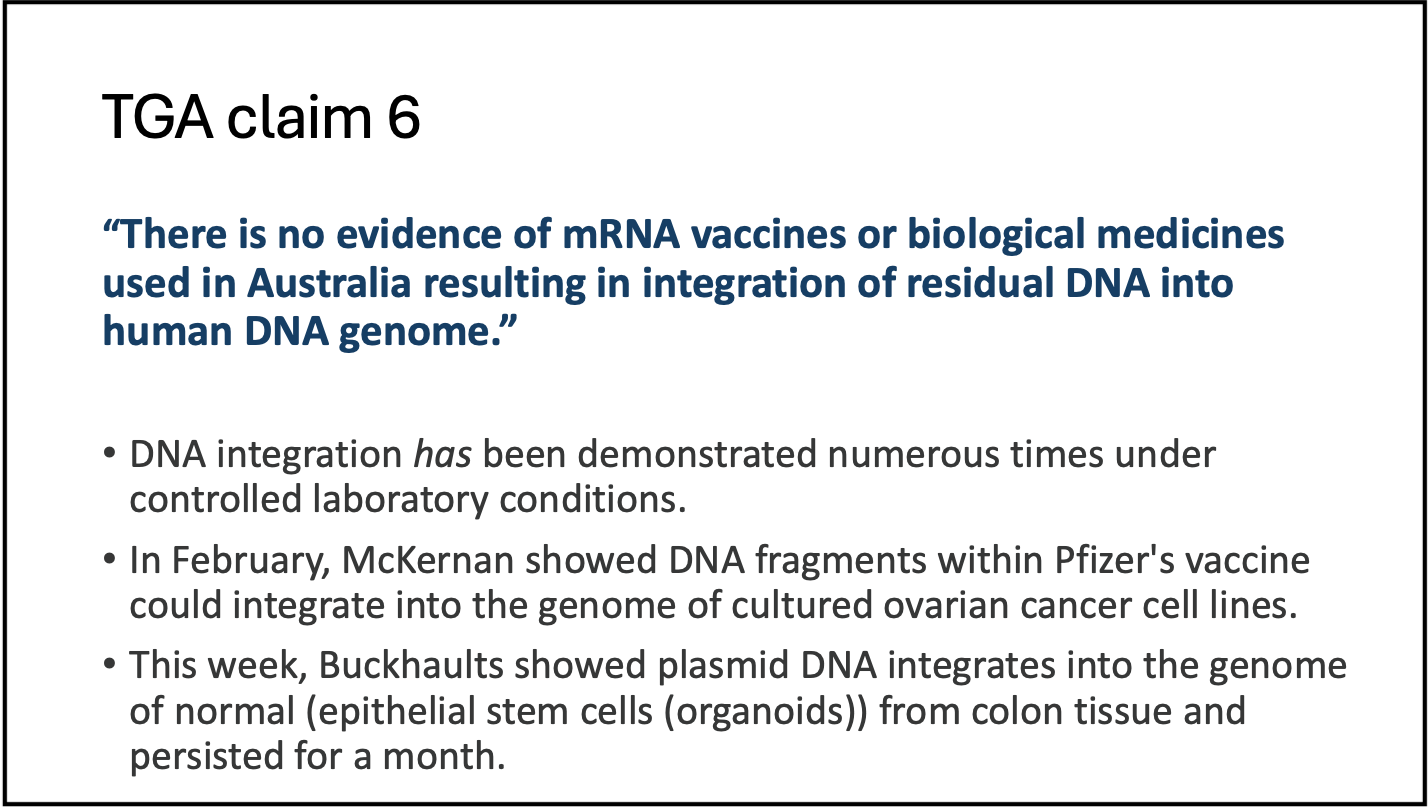
Finally, the TGA said there’s no evidence that the residual DNA in these vaccines can lead to integration of the human genome. However, it has been demonstrated numerous times under controlled laboratory conditions.
In February, Kevin McKernan showed that DNA fragments in Pfizer’s vaccine could integrate into the genome of ovarian cancer cell lines.
Kevin received criticism for this because cancer cell lines in the lab are “immortal” and do not represent normal human cells.
So, Professor Buckhaults took healthy human epithelial stem cells from the colon and mixed them with mRNA vaccines and showed that integration persisted in these cells for a month. He just released these results this week.
Both researchers are currently looking for DNA integration in the tumours of people who have been vaccinated - which should be the job of the regulator, but instead is left to independent researchers to do the work.
The TGA released its own data recently and said that all the levels of residual DNA in the 28 batches it tested, complied with the WHO’s recommended limit of 10 nanogram per dose. However, this did not quell concerns.
None of the batches tested by the TGA were registered prior to 2023 and therefore did not represent batches that were used to vaccinate the vast majority of Australians.
Further, we know that later batches were “cleaner” because Buckhaults looked at batches manufactured in 2023 and found they had 10 times less residual DNA than batches from 2020.
This leaves us with some important questions.
What are the levels of residual DNA in the vaccines that were used to inject the majority of Australians? This is important to know because if there is a ‘bad batch’ we may be able to link it to people who’ve had the most severe vaccine injuries.
Second, given the presence of lipid nanoparticles, which are “transfection agents” that transport genetic material inside cells, what are the clinical implications of having even small amounts of residual DNA in these products?
Where are the DNA integration studies by drug regulators? They have not produced any evidence to date.
And finally, what are the cancer rates in people who have been vaccinated with these products? We know from our FOI that there are no data - so who is looking?
I’ll leave it there, and thank you for listening.
NB: Questions were presented in a panel discussion at the end of the night.