Stairs to Nowhere: TGA’s vanishing promise of safety monitoring
A four-year paper trail of contradictions: how Australia’s drug regulator promised transparency on Covid-19 vaccine safety, then lost track of its own oversight.
“A true democracy is a place of many voices, including discordant voices.”
— Justice Michael Kirby
GUEST POST
P. Rekaris is an independent researcher specialising in regulatory transparency.
Julie Sladden is a medical doctor (retired), writer, and Local Government Councillor with a passion for transparency in healthcare and regulation.
We first reported on this issue in May 2025. When Australia rolled out COVID-19 vaccines, Australia’s drug regulator, the Therapeutic Goods Administration (TGA) promised enhanced safety monitoring.
Four years later, they admit they never systematically tracked whether they kept that promise.
What began as a straightforward request for transparency has resulted in extraordinary contradictions.
This article provides an updated account through November 2025, including new Senate testimony that reinforces a disturbing and persistent pattern.
The promise
In February 2021, the TGA published a COVID-19 Vaccine Safety Monitoring Plan.
Because the COVID-19 vaccines received only “provisional” approval based on limited data, the TGA was legally required to conduct enhanced safety monitoring beyond normal surveillance.
The plan wasn’t optional. It was a legal requirement for provisional approval.
The request
In February 2022, we submitted a straightforward Freedom of Information (FOI) request: show us a single document proving this safety monitoring plan was implemented.
This was reasonable because provisional approval requires enhanced monitoring beyond routine surveillance, and Australia’s therapeutic goods regulator needs to demonstrate these conditions are being met.
The TGA’s response? “The document does not exist.”
But when challenged through the Information Commissioner in February 2022, the TGA admitted it held “ample documentation” relating to the plan.
A senior TGA official backpedalled stating, “We would of course have ample documents demonstrating things we have done that correspond to each of the objectives of the plan.”
Scrutiny by the Senate – the startling contradiction
In October 2025 Senate Estimates, Senator Alex Antic pressed the issue.
Senior TGA official Dr Daniel Dascombe told the Senate that a “vast volume” of documents exist – contradicting the original FOI response.
He added that producing these documents would be difficult because the safety monitoring was just “day-to-day processes” that were never systematically tracked by the plan’s objectives.
Wait… The TGA conducted ‘enhanced’ safety monitoring by treating it as routine ‘day-to-day’ work?
That is not what provisional approval requires.
Provisional approval is contingent on demonstrable, trackable enhanced monitoring – not merely standard “business as usual”. Without separate tracking and reporting, compliance cannot be proven.
Moreover, how can enhanced provisional monitoring become “day-to-day processes” without any documented transition?
Later, TGA head Professor Tony Lawler told the Senate the agency was “very happy to receive any requests for freedom of information,” while simultaneously defending the refusal to release documents.
Earlier in August, Senator Antic’s attempts to obtain these documents through Parliament met a brick wall.
Senator Katy Gallagher dismissed the motion as “another trawling expedition from an anti-vaccination Senator” and said production would be an “unacceptable diversion of departmental resources.”
This response is troubling.
Parliamentary oversight is a constitutional duty under section 49 – not a “trawling expedition.” Senators must scrutinise the executive, especially on public health affecting millions.
If the regulator fulfilled its monitoring obligations, why would transparency cripple operations?
The pattern: what the TGA can do – and claims it can’t
In September 2024, the TGA demonstrated that it could, in fact, organise documents.
When reporting to the Information Commissioner, it systematically classified hundreds of documents into 12 categories: Standard Operating Procedures, protocols, workflows, electronic briefings, and more.
We took note.
The TGA identified 399 documents, totalling nearly 4,000 pages. In April 2025, we requested just 30 of them – a modest 7.5% – using the TGA’s own classification system.
We thought this was reasonable.
The TGA approved 5 documents as “clear and reasonable” but rejected the rest, claiming the request was too “subjective” and would “unreasonably divert resources.”
In other words, the same system that organised hundreds of documents for the Information Commissioner suddenly became unworkable for 7.5% of what they admitted holding.
This illustrates bureaucratic confusion, not mere systematic obstruction.
Over four years, a clear pattern has emerged.
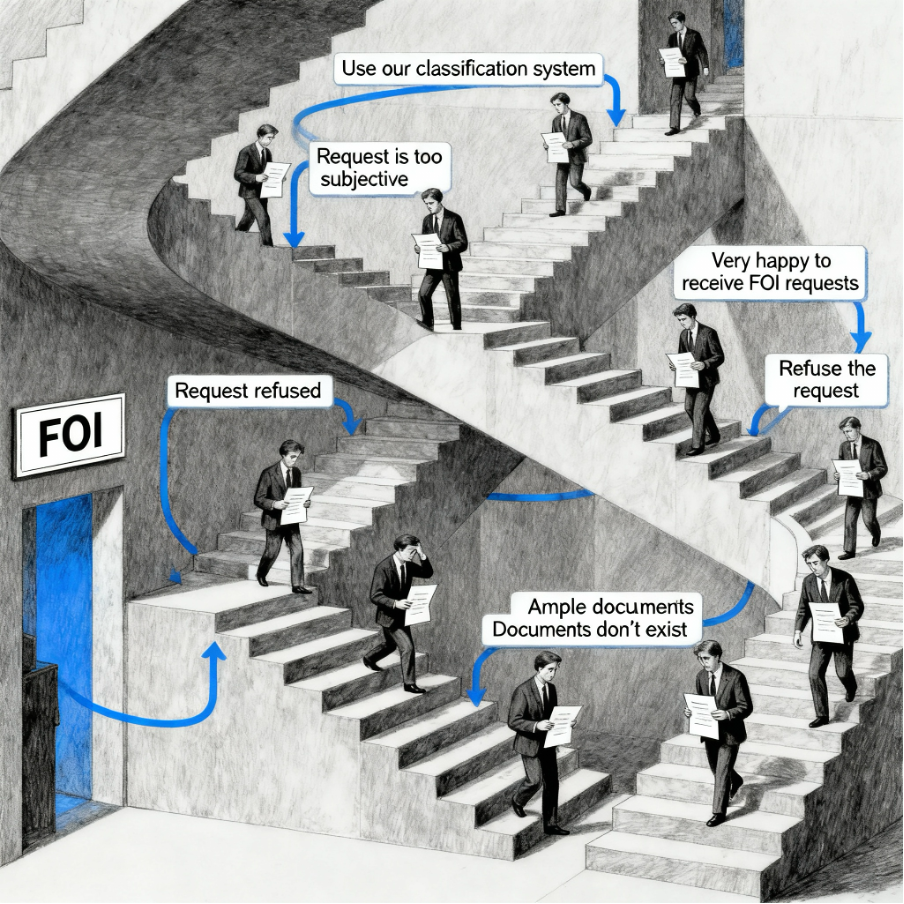
Escher’s stairs: a FOI process that leads nowhere
The FOI process has come to resemble an Escher drawing – a staircase that appears to lead somewhere but ultimately goes nowhere. This is our lived experience across nearly 1,400 days of trying to verify basic regulatory oversight.
Each time we follow the TGA’s FOI instructions, we climb another flight of impossible stairs:
February 2022: “Documents don’t exist”
July 2022: “Ample documentation exists”
September 2024: Successfully organises thousands of documents for the Commissioner
June 2025: Can’t use the same system for citizens – it’s too “subjective”
October 2025: “vast volumes” exist, but producing them would be too difficult. Yet, “very happy to receive FOI requests”
Like Escher’s famous staircase, we keep climbing, but we never arrive anywhere.
Why this matters
Three reasons make this far more than a paperwork dispute:
Legal requirements: Provisional approval required enhanced monitoring beyond routine work. If monitoring was just ‘day-to-day processes,’ were the legal conditions actually met?
Accountability: The TGA made a public promise in February 2021. Four years later, it cannot verify it systematically kept that promise.
Transparency standards: The TGA can organise documents when reporting to regulators but claim it’s impossible when citizens ask.
This pattern reveals a systemic issue: if an agency cannot systematically verify its own safety plan objectives, does it possess the basic organisational capability necessary for effective governance?
The TGA’s admission that key pharmacovigilance documents are not organised according to safety plan objectives directly contradicts the international guidelines which Australia has adopted, and could constitute breaches of legislative Acts governing public governance and records management.
These unresolved matters raise important questions not just regarding safety monitoring for COVID-19 vaccines, but potentially for all provisionally approved pharmaceuticals.
The question nobody can answer
If enhanced monitoring was conducted as legally required, why can’t the TGA produce straightforward documentation?
There are only three possible explanations:
The monitoring wasn’t conducted as a structured programme
Records are too disorganised to retrieve
Documents were never systematically maintained as required
Every explanation points to regulatory failure.
What’s really at stake
Millions of Australians received provisionally approved vaccines under policies that cost people their livelihoods, divided families, and restricted access to society.
People made life-altering decisions based on assurances that robust safety monitoring was in place. Four years later, the regulator cannot prove it met that legal obligation.
The unanswered question
Despite hundreds of pages of correspondence, two Information Commissioner reviews, multiple consultations with the TGA, Senate questions, and an Ombudsman complaint, Australians still don’t know whether the safety monitoring they were promised was ever implemented.
This matter is now before the Information Commissioner yet again, with the Commonwealth Ombudsman investigating.
The TGA admits it holds nearly 4,000 pages of documents but still refuses to release them.
If there’s “no difficulty” demonstrating pharmacovigilance – as TGA leadership claims – then why can’t those documents be produced to the Australian public?
Australians deserve answers and transparency from an agency entrusted with their safety.
Note: This article is based on documented FOI correspondence, TGA submissions to the Information Commissioner, and Senate Community Affairs Legislation Committee testimony spanning 2022–2025. Key evidence includes TGA OAIC Submission MR22/00538 (September 2024), FOI 25-0166 Decision (June 2025), and Senate Community Affairs Legislation Committee Hansard (9 October 2025). Matter Reference: MR25/01153 (FOI 25-0166)
Document Archive: [LINK]



Maryanne, Very Australian. Always allowing that it is just confusion or bumbling that is leading to nothing working the way it should. I would suggest that these are evil people doing evil things would be a far more credible (and likely correct) explanation for everything exposed here.
Katy Gallagher is disgusting. In parliament even last year she repeated the total lie that the unvaxxed were 30 times more likely to pass on covid - a claim which has never ever been backed up, and as we know, is completely false based on study after study we have available.
But I digress. Katys claims about time wasting are completely wrong. If there is a process that has to be followed then it needs to be followed. Yes, the TGA would love to do just what it wants to do, but this is what it NEEDS to do. And if people and depts don't do what they NEED to do then politicians should not provide cover for them.
Australia is in a very bad place where an ever expanding horde of unelected officials carry out largely wasteful and unneeded actions, and refuse to be held accountable. If this were a listed company the board would have been sacked and the share price would be in free fall as governance had completely failed - but because its a govt dept they apparently can do whatever they want.
Basically we need a Reformation of Australia to completely recast our systems so that the TGA and others cannot wilfully flout legal and ethical requirements. In fact I would go so far as to ask why we even need a TGA anyway.